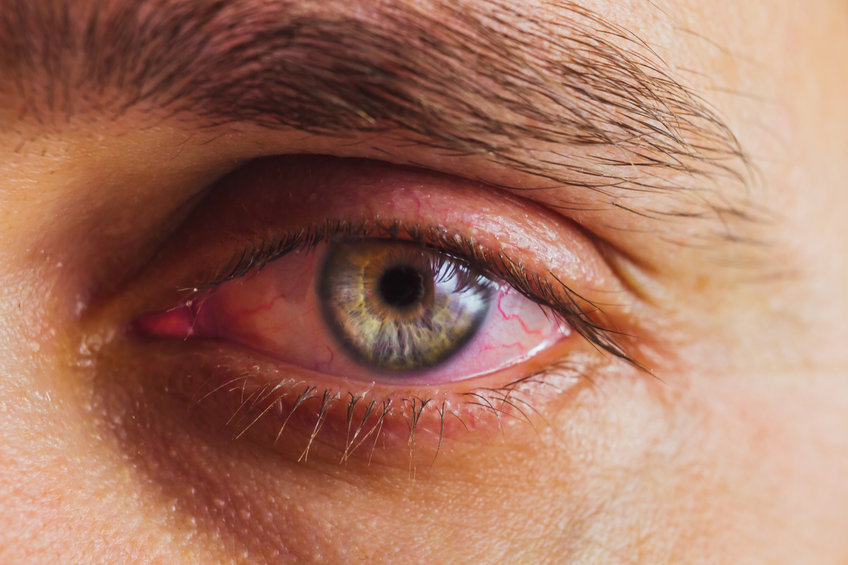
Restasis (Cyclosporine Ophthalmic Emulsion) 0.05% was released by Allergan in 2003 for the treatment of dry eye disease. For the last 20 years, Restasis has been available as brand name only, until now. In February 2022, the FDA approved the first generic version of Restasis.
Dry eye disease has 3 main categories; evaporative, aqueous (water) deficient, and inflammatory. Restasis is thought to act on the inflammatory pathway, promoting natural tear production. Restasis can be used alone or in combination with other dry eye therapies. Restasis is particularly effective in patients with inflammatory conditions like Sjogren’s that affect the tear-producing glands. When used twice daily, patients can usually expect to notice a benefit within 90 days. Common side effects include eye redness and stinging upon instillation, though Restasis is generally well tolerated.
Cyclosporine Ophthalmic Emulsion 0.05% has been proven to be effective in clinical trials, increasing tear production, and improving patient comfort. Although this has been well accepted and adopted by the eyecare community, a major hurdle in getting the drug to patients has been cost. In many cases, patients are limited by their insurance companies benefits and drug formularies. Without evidence from the provider that this medication was absolutely necessary, Restasis would often be rejected. In some cases, brand name medications like Restasis simply aren’t covered at all, leaving the patient to either pay out of pocket, or try a different medication.
Now that Restasis is available as a generic, that major hurdle has been removed. Not only is Restasis an expensive medication with variable insurance coverage, as stated above, it can take up to 3 months of regular use before the patient experiences any benefit. With this medication now removed from its brand, patients should have greater access to the therapeutic benefits realized by this medication 20 years ago. Many clinicians, myself included, leave drops like Restasis as a plan “C” or “D”, simply due to the cost associated with them. Restasis is not the only medication in this category. Two other newer drops, Xiidra and Cequa, work on the eyes in similar ways, however, are both still brand names. Programs are in place by these companies to help lower costs for patients, but the process can be complex and inconsistent.
Branding of medication and the cost associated with the production of new medications is in some ways a necessary consequence of medical advancement. Bringing a new drug to market costs an enormous amount of money in research and development (R& D), and companies like Allergan maintain their patents to recoup the investment in that R& D. However, while understanding the process, it is an update that patients and doctors alike should be excited about.
Cyclosporine Ophthalmic Emulsion is still likely to come with an out-of-pocket cost and face some opposition from insurance companies. However, this change will certainly benefit patients and their ultimate care. If you have questions regarding dry eye or if you think this medication could be right for you, talk to your doctor. See the links below for more information.
References:
https://www.fda.gov/news-events/press-announcements/fda-approves-first-generic-restasis