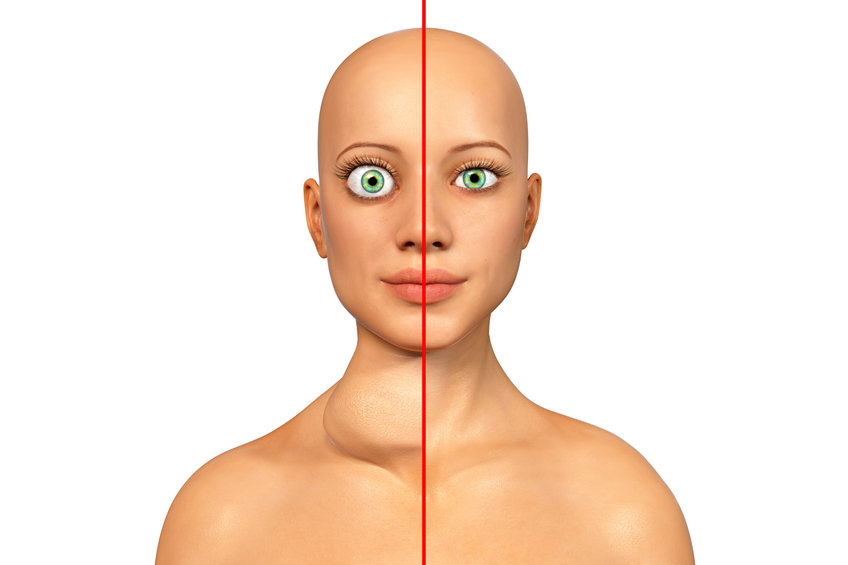
Thyroid Eye Disease (TED) is an autoimmune condition affecting approximately 1% of adults. TED is characterized by increased tissue volume surrounding the eyes. Symptoms initially include dryness and irritation, but can evolve to include double vision and even blindness. Most commonly, TED is associated with hyperthyroidism, or too much thyroid hormone circulating in the blood. You may have also heard of hyperthyroidism referred to as Graves. Although TED is associated with this condition, it can occur on its own and in the presence of normal, or even low thyroid levels. Historically, management of TED was primarily directed at identifying and treating the underlying cause. However, as discussed, not all cases are as cut and dry, which has left many patients stuck with invasive surgical procedures to relieve their symptoms.
Read our blog about Thyroid Eye Disease
Introducing Tepezza, the first FDA approved medication for TED. Tepezza is in a drug class known as “biologics”, similar to Humira. These medications regulate our immune systems, helping to manage the damage associated with autoimmune conditions. Tepezza is an infusion, which means the medication is slowly administered intravenously (IV) by a healthcare professional. The treatment course takes place over six months and includes one infusion every three weeks for a total of eight infusions. In a clinical study, Tepezza was shown to decrease eye bulging, improve symptoms of double vision, and reduce the appearance of redness and swelling. These improvements were experienced by some patients in as little as six weeks, and a majority of patients in the study maintained an improvement in symptoms past one year.
Side effects of Tepezza include muscle spasms, nausea, hair loss, diarrhea, fatigue, hearing impairment, altered taste, headache, dry skin, and menstrual disorders. Infusion reactions also affected a small percentage of patients, but were usually mild to moderate in severity. Tepezza can also affect your blood sugar, causing it to increase. This is especially important if you have diabetes or are prediabetic. Tepezza may worsen existing inflammatory bowel diseases (IBD), so it is important to disclose your full medical history to your healthcare provider prior to starting Tepezza. Report any side effects or reactions you think you may be having to your doctor. For more information, talk to your doctor, and see the links below.
References:
https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-thyroid-eye-disease
https://www.medicalnewstoday.com/articles/drugs-tepezza#about