In recent decades, there has been a national crisis in the United States of deaths caused by opioid overdoses. This has been labeled the opioid epidemic. Opioids are drugs that are used to treat pain. There are prescription opioids such as oxycodone (OxyContin), hydrocodone (Vicodin), and morphine. Fentanyl and methadone are in a subclass of opioids called synthetic opioids. Fentanyl can be prescribed legally, however illegally made and distributed fentanyl has been seen in increasing amounts in the United States. Then, there’s heroin which is an illegal opioid. When taken in doses that are too high or when taken with certain other drugs or substances, opioids can cause fatal overdose. The National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC) uses the State Unintentional Drug Overdose Reporting System (SUDORS) to record and report deaths due to drug overdoses.
Read our blog on Treating Chronic Pain More Safely
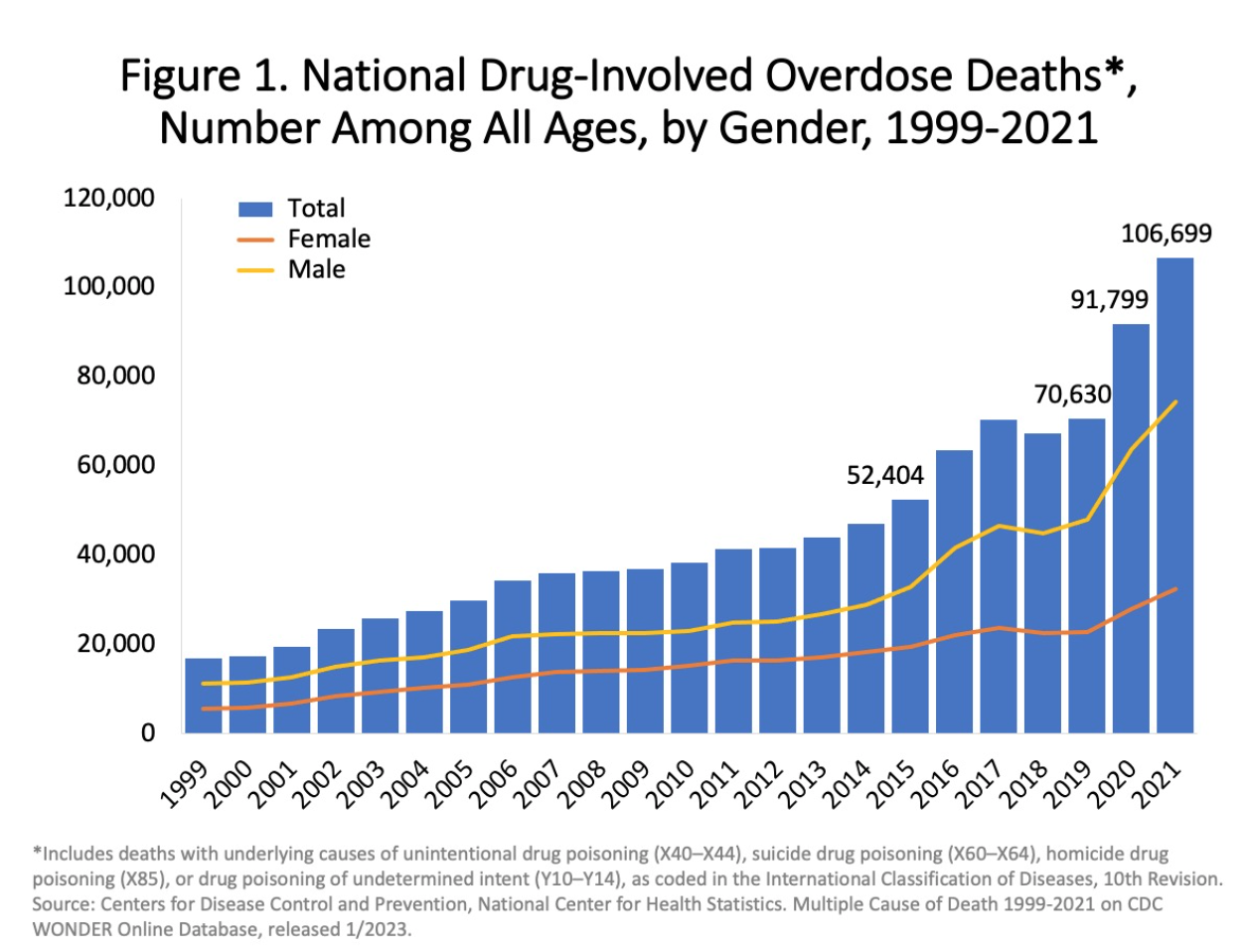
According to the CDC, more than 106,000 people in the U.S. died from drug related overdoses in 2021, including both illicit drugs and prescription opioids. 75% of overdoses involved an opioid in 2020. Despite efforts to improve opioid prescribing and public education campaigns, the opioid epidemic is worsening. From 2019 to 2020, opioid-involved death rates increased by 38%, which includes a 17% increase in prescription opioid-involved deaths, and an alarming 56% increase in deaths related to synthetic opioids (excluding methadone), primarily fentanyl.
Naloxone
Given the tremendous death toll and social destruction attributed to opioids, critical steps have been taken in an effort to reduce opioid overdose deaths. One of these steps has been a national push to widely distribute the opioid reversal agent, naloxone, amongst communities. Naloxone, often known by the brand name Narcan, is a medication that can reverse the dangerous effects of opioids. When given in time, naloxone can be a life-saving medication in the setting of overdose from any opioid, including heroin, fentanyl, and prescription opioids. Naloxone is available in two forms: a prefilled syringe and a nasal spray.
The newest piece of the crucial naloxone public health initiative was changing the status of naloxone from prescription only to over-the-counter. Over-the-counter status increases access to this medication and therefore potentially saves more lives by preventing fatal overdoses. Naloxone nasal spray was first approved by the U.S. Food and Drug Administration (FDA) in 2015 as a prescription medication. On March 29, 2023 the FDA announced the approval of Narcan nasal spray for over-the-counter, non-prescription use. This is the first naloxone product to ever be approved over-the-counter. In order for the drug status to be changed from prescription to over-the-counter, the manufacturer was required to provide evidence to the FDA that consumers can understand how to use naloxone safely and effectively without the supervision of a healthcare professional. The application for over-the-counter status of Narcan was granted priority status and reviewed by an advisory committee in February 2023. The committee unanimously voted to approve the change in status. Availability and price of over-the-counter Narcan nasal spray will be determined by the manufacturer, Emergent BioSolutions Inc., who is reporting to expect Narcan on shelves by late summer.
For more information on how to use naloxone, visit the CDC website here: https://www.cdc.gov/stopoverdose/naloxone/index.html and/or download the OpiRescue app here: https://www.rxapp.link/.
Resources:
- https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates
- https://www.cdc.gov/opioids/basics/index.html
- https://www.cdc.gov/opioids/basics/epidemic.html
- https://www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-nalox one-nasal-spray
- https://www.wsj.com/articles/fda-approves-narcan-for-non-prescription-sale-8386c3e
- https://www.cdc.gov/stopoverdose/naloxone/index.html
- https://www.rxapp.link/